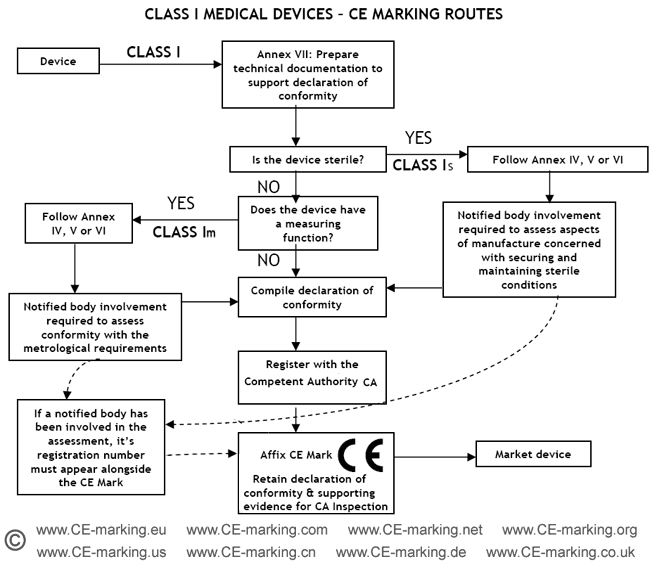
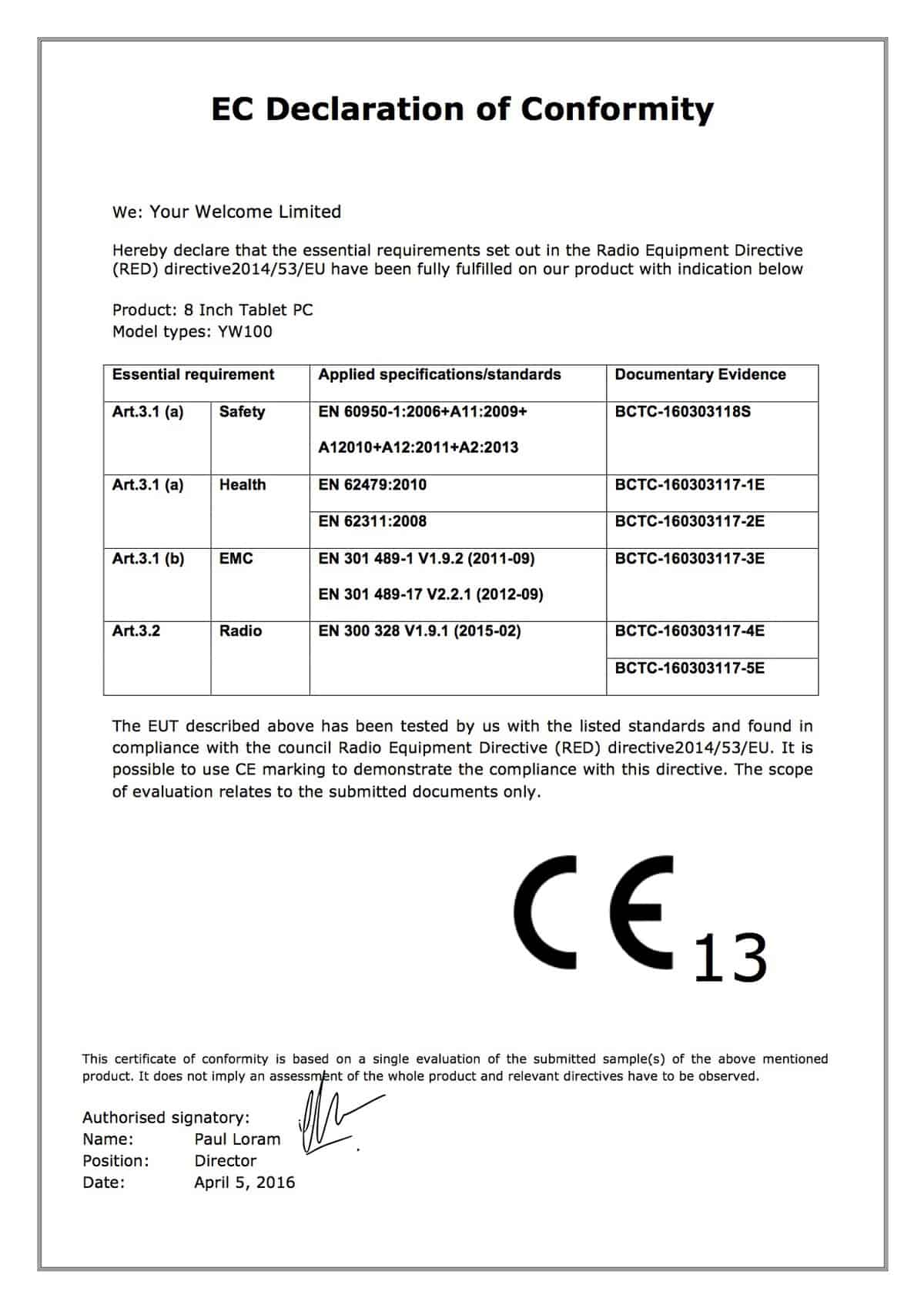
Ce Declaration Of Conformity Template
Ce declaration of conformity template - I know when you read, the requirements, this looks easy. The word “declaration of conformity” is written 38 times on the eu mdr 2017/745. In short, make sure you have your declaration of conformity in place before you start importing and selling ce products in the eu. As mentioned, amazon may also suspend your product listings. The declaration of performance is a key part of the construction products regulation. The gspr is known as general safety and performance requirements are listed in annex i of eu mdr 2017/745 and eu ivdr 2017/746.they are similar to the essential requirements under mdd 93/42/eec. Standards are developed by private standardisation organisations usually on the initiative of stakeholders. This is, if i may say, a pillar on the medical device regulation process. It’s just a document that you sign to congratulate yourself of the great job you have done and to swear that you have respected all the laws. The gspr has 23 requirements under mdr and 20 requirements under ivdr.the manufacturers who want to get their device ce marked have to.
Each construction product covered by a european harmonised standard or for which a european technical assessment has been issued needs this declaration and has to be ce marked. It provides information on the performance of a product.
Example of a declaration of conformity CE Marking assistant
Each construction product covered by a european harmonised standard or for which a european technical assessment has been issued needs this declaration and has to be ce marked. The gspr has 23 requirements under mdr and 20 requirements under ivdr.the manufacturers who want to get their device ce marked have to. As mentioned, amazon may also suspend your product listings.
Guide on Class I (Is/Im) MDD Medical Devices CE marking (mark
The declaration of performance is a key part of the construction products regulation. This is, if i may say, a pillar on the medical device regulation process. Each construction product covered by a european harmonised standard or for which a european technical assessment has been issued needs this declaration and has to be ce marked.
Declaration of Conformity
This is, if i may say, a pillar on the medical device regulation process. As mentioned, amazon may also suspend your product listings. I know when you read, the requirements, this looks easy.
EU Declaration of Conformity Parallel Wireless
It provides information on the performance of a product. I know when you read, the requirements, this looks easy. As mentioned, amazon may also suspend your product listings.
Declarations of conformity O'Dive
As mentioned, amazon may also suspend your product listings. It provides information on the performance of a product. Each construction product covered by a european harmonised standard or for which a european technical assessment has been issued needs this declaration and has to be ce marked.
Quality Certifications, ISO 90012015 Dynex
Each construction product covered by a european harmonised standard or for which a european technical assessment has been issued needs this declaration and has to be ce marked. Standards are developed by private standardisation organisations usually on the initiative of stakeholders. The declaration of performance is a key part of the construction products regulation.
Certificate Of Conformity Uk Template HQ Template Documents
In short, make sure you have your declaration of conformity in place before you start importing and selling ce products in the eu. It provides information on the performance of a product. The gspr is known as general safety and performance requirements are listed in annex i of eu mdr 2017/745 and eu ivdr 2017/746.they are similar to the essential requirements under mdd 93/42/eec.
Declaration of Conformity FIG 181/182 CI ANG/GLB BODY
It provides information on the performance of a product. The word “declaration of conformity” is written 38 times on the eu mdr 2017/745. The gspr has 23 requirements under mdr and 20 requirements under ivdr.the manufacturers who want to get their device ce marked have to.
It provides information on the performance of a product. The word “declaration of conformity” is written 38 times on the eu mdr 2017/745. The declaration of performance is a key part of the construction products regulation. The gspr is known as general safety and performance requirements are listed in annex i of eu mdr 2017/745 and eu ivdr 2017/746.they are similar to the essential requirements under mdd 93/42/eec. It’s just a document that you sign to congratulate yourself of the great job you have done and to swear that you have respected all the laws. This is, if i may say, a pillar on the medical device regulation process. The gspr has 23 requirements under mdr and 20 requirements under ivdr.the manufacturers who want to get their device ce marked have to. Each construction product covered by a european harmonised standard or for which a european technical assessment has been issued needs this declaration and has to be ce marked. As mentioned, amazon may also suspend your product listings. I know when you read, the requirements, this looks easy.
Standards are developed by private standardisation organisations usually on the initiative of stakeholders. In short, make sure you have your declaration of conformity in place before you start importing and selling ce products in the eu.