Irb Protocol Template
Irb protocol template - The irb will be responsible for documenting that the activities proposed involve greater than minimal risk. Faqs and guidance reliance policies meeting dates please contact us with any questions! A companion protocol template for exempt research may be found in the feature box, related information (top right). The irb will continue to treat minimal risk activities as expedite review and will work to educate staff and researcher on the interpretation of the expedite categories. Human gene therapy review procedures (ppt) other resources. If wcg irb has not previously approved the protocol, submit the sponsor’s template as a microsoft word compatible file. Application irb protocol, no contact with subjects; Irb concerns with site submission information: The nih review process for human gene transfer trials; Emergency exemption from prospective irb approval.
See all clinical research news filter this list learn how this search works. Even in situations where the irb may waive the documentation (signature) requirement (e.g., telephone interview, online survey), investigators are expected to present participants with the required key elements of informed consent. Nih notice march 22, 2016 Irb application form by protocol type expedited/full board (accessible 08/16/22) exempt (accessible 08/16/22) And (b) the characteristics of the participant population being studied;
Research Protocol & IRB Instructions tampaERdoc
Application irb protocol, no contact with subjects; Nih notice march 22, 2016 The form guides are organized by protocol type and submission type.
IRB Software Management & Compliance Tracking System
Application irb protocol, no contact with subjects; Human gene therapy review procedures (ppt) other resources. Irb application form by protocol type expedited/full board (accessible 08/16/22) exempt (accessible 08/16/22)
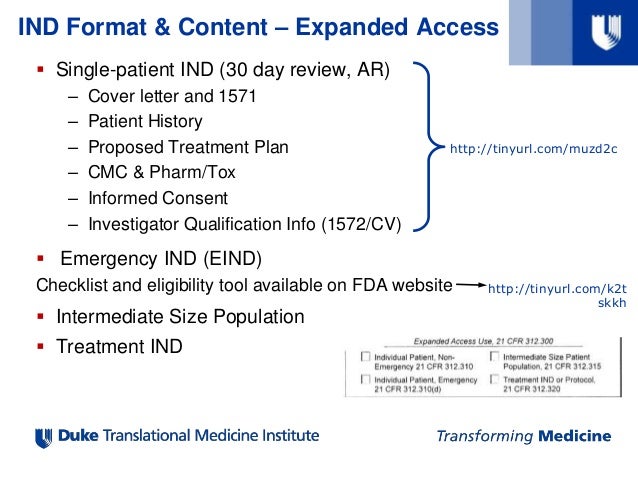
IND Application Process and Best Practices
The irb will continue to treat minimal risk activities as expedite review and will work to educate staff and researcher on the interpretation of the expedite categories. Additional information on creating and submitting an irb application in kuali protocols can be found in the kuali protocols instructional guides. If wcg irb has not previously approved the protocol, submit the sponsor’s template as a microsoft word compatible file.
Writing a Protocol CHOP Institutional Review Board
In addition, the biological sciences division of the university of chicago. See all clinical research news filter this list learn how this search works. And (b) the characteristics of the participant population being studied;
CTN Webinar Impact of Inclusion and Exclusion Criteria on Study
The irb will be responsible for documenting that the activities proposed involve greater than minimal risk. The complete data and safety monitoring report template should be included as an appendix. The form guides are organized by protocol type and submission type.
Designing the Study CHOP Institutional Review Board
Application irb protocol, no contact with subjects; Upon review of additional sites conducting a previously approved protocol, the irb may note an area of concern with the site’s responses on the submission documents. If concerns are noted or the compensation for participation information is unclear, an advarra representative will contact the site.
PPT Orientation for New Clinical Research PERSONNEL Module 2
The complete data and safety monitoring report template should be included as an appendix. A companion protocol template for exempt research may be found in the feature box, related information (top right). Tips for filling out zipline applications;
Informed Consent Template by Pharma Student Issuu
See all clinical research news filter this list learn how this search works. The form guides are organized by protocol type and submission type. In addition, the biological sciences division of the university of chicago.
Irb application form by protocol type expedited/full board (accessible 08/16/22) exempt (accessible 08/16/22) Tips for filling out zipline applications; The irb will continue to treat minimal risk activities as expedite review and will work to educate staff and researcher on the interpretation of the expedite categories. In addition, the biological sciences division of the university of chicago. Emergency exemption from prospective irb approval. Please review for important guidance related to reporting protocol deviation/violations to the irb related to the global omnipaque shortage. Continuing review requirement for expedited, minimal risk research Application irb protocol, no contact with subjects; Federalwide assurance (fwa) federal regulations require an irb to review research on human subjects if the research involves federal funding. Human gene therapy review procedures (ppt) other resources.
Irb concerns with site submission information: The irb will be responsible for documenting that the activities proposed involve greater than minimal risk. Upon review of additional sites conducting a previously approved protocol, the irb may note an area of concern with the site’s responses on the submission documents. If wcg irb has not previously approved the protocol, submit the sponsor’s template as a microsoft word compatible file. Additional information on creating and submitting an irb application in kuali protocols can be found in the kuali protocols instructional guides. A companion protocol template for exempt research may be found in the feature box, related information (top right). Nih notice march 22, 2016 See all clinical research news filter this list learn how this search works. If concerns are noted or the compensation for participation information is unclear, an advarra representative will contact the site. Faqs and guidance reliance policies meeting dates please contact us with any questions!
The complete data and safety monitoring report template should be included as an appendix. The form guides are organized by protocol type and submission type. And (b) the characteristics of the participant population being studied; Even in situations where the irb may waive the documentation (signature) requirement (e.g., telephone interview, online survey), investigators are expected to present participants with the required key elements of informed consent. The nih review process for human gene transfer trials;