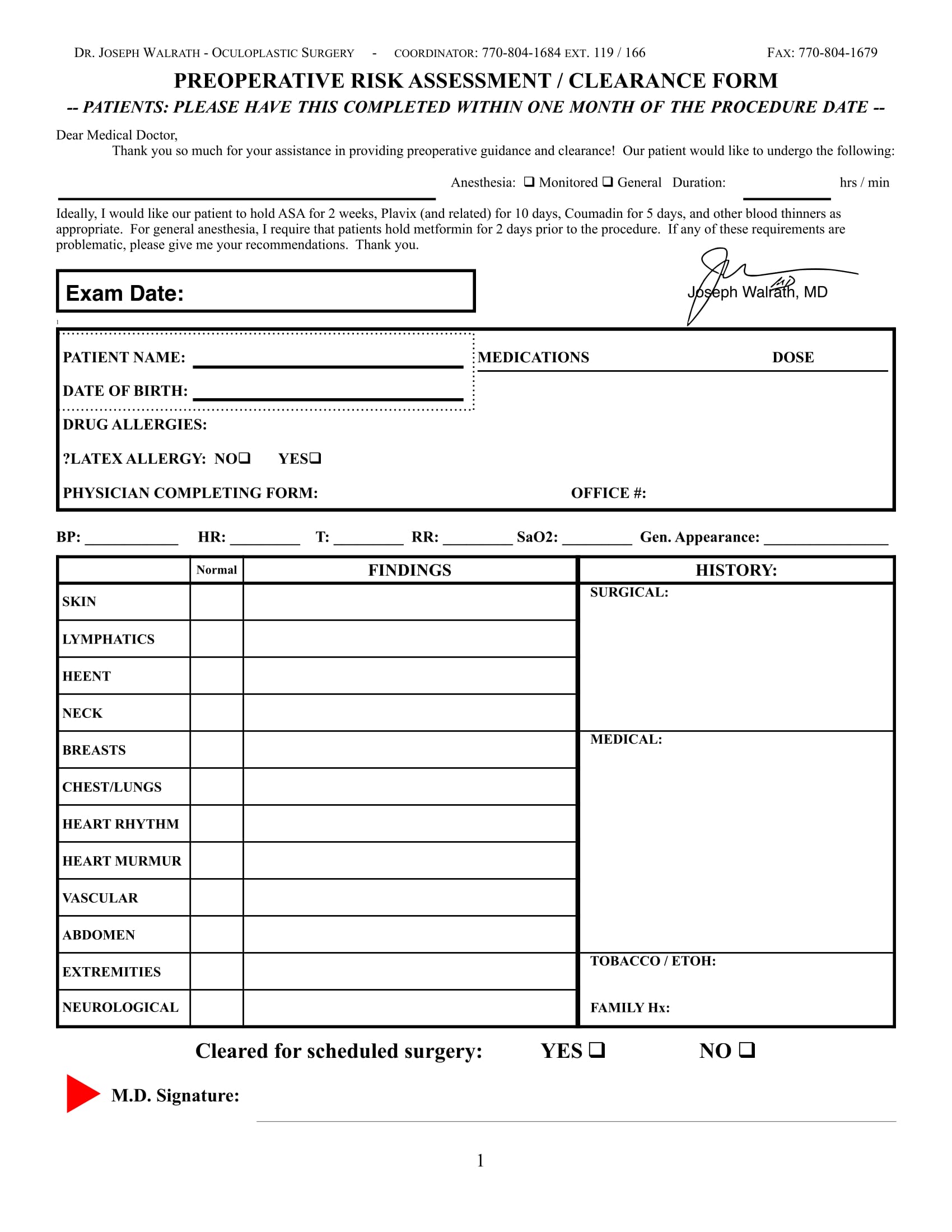
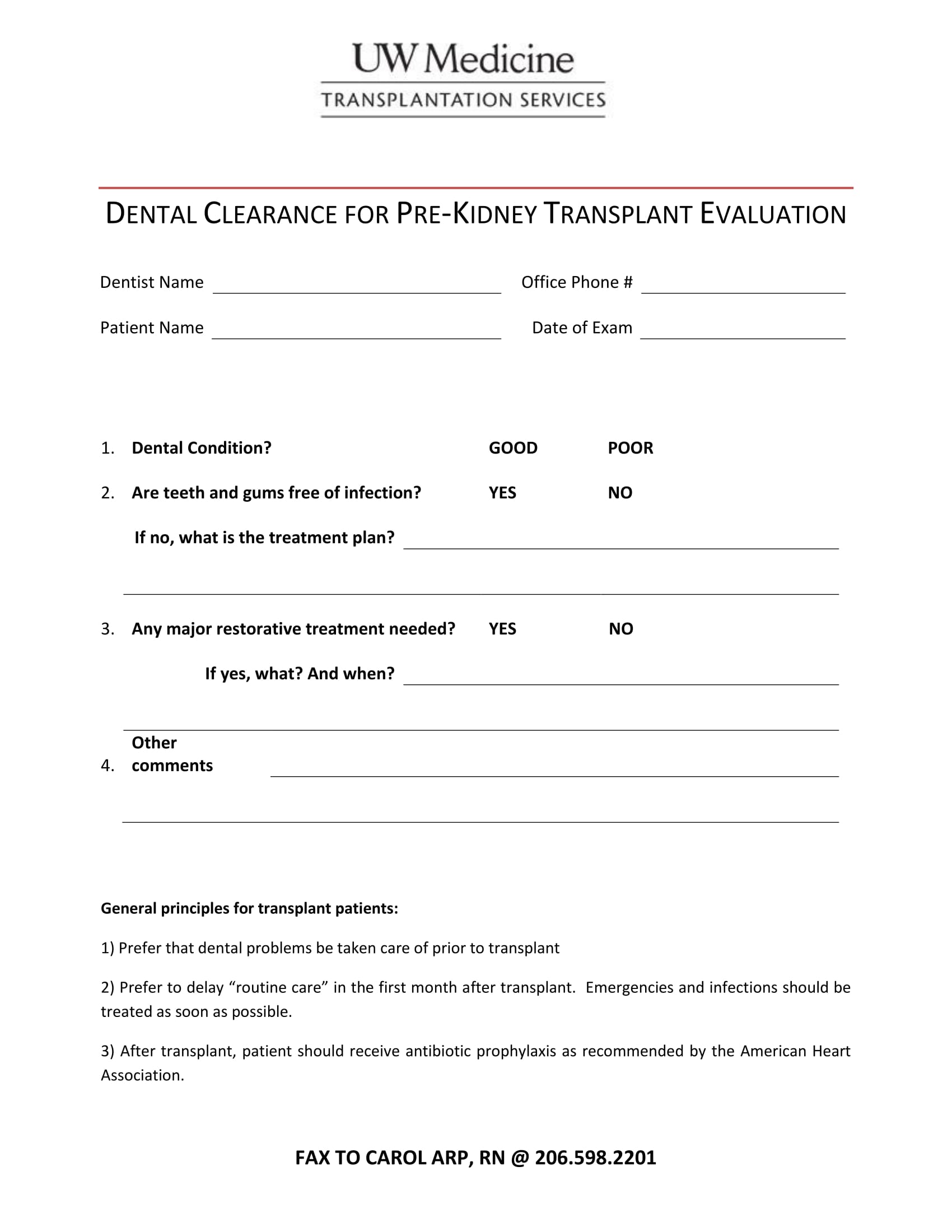
Pre Op Clearance Template
Pre op clearance template - Food and drug administration (fda) to oversee the safety of food, drugs, medical devices, and cosmetics.a principal author of this law was royal s. + 421 2 507 01 206. Tlačivo na hlásenie nežiaduceho účinku je na webovej stránke. Štátny ústav pre kontrolu liečivsekcia klinického skúšania liekov a farmakovigilanciekvetná ul. Your device is exempted from 510(k) by regulation. Once the foreign manufacturer has received 510(k) clearance for the device, the foreign manufacturer may export his device to any u.s. The united states federal food, drug, and cosmetic act (abbreviated as ffdca, fdca, or fd&c) is a set of laws passed by the united states congress in 1938 giving authority to the u.s.
preop clearance form Heart.impulsar.co
Štátny ústav pre kontrolu liečivsekcia klinického skúšania liekov a farmakovigilanciekvetná ul. + 421 2 507 01 206. Food and drug administration (fda) to oversee the safety of food, drugs, medical devices, and cosmetics.a principal author of this law was royal s.
FREE 31+ Medical Clearance Forms in PDF MS Word
Štátny ústav pre kontrolu liečivsekcia klinického skúšania liekov a farmakovigilanciekvetná ul. Food and drug administration (fda) to oversee the safety of food, drugs, medical devices, and cosmetics.a principal author of this law was royal s. Your device is exempted from 510(k) by regulation.
FREE 29+ Sample Medical Clearance Forms in PDF Word Excel
+ 421 2 507 01 206. Štátny ústav pre kontrolu liečivsekcia klinického skúšania liekov a farmakovigilanciekvetná ul. The united states federal food, drug, and cosmetic act (abbreviated as ffdca, fdca, or fd&c) is a set of laws passed by the united states congress in 1938 giving authority to the u.s.
Anesthesiologist Assistant Student Primer Preop evaluation
Tlačivo na hlásenie nežiaduceho účinku je na webovej stránke. Food and drug administration (fda) to oversee the safety of food, drugs, medical devices, and cosmetics.a principal author of this law was royal s. Your device is exempted from 510(k) by regulation.
Medical Clearance May 5 2011
Tlačivo na hlásenie nežiaduceho účinku je na webovej stránke. + 421 2 507 01 206. Štátny ústav pre kontrolu liečivsekcia klinického skúšania liekov a farmakovigilanciekvetná ul.
FREE 32+ Clearance Form Examples in PDF MS Word
The united states federal food, drug, and cosmetic act (abbreviated as ffdca, fdca, or fd&c) is a set of laws passed by the united states congress in 1938 giving authority to the u.s. + 421 2 507 01 206. Once the foreign manufacturer has received 510(k) clearance for the device, the foreign manufacturer may export his device to any u.s.
9 Medical Clearance Form Download for Free Sample Templates
Štátny ústav pre kontrolu liečivsekcia klinického skúšania liekov a farmakovigilanciekvetná ul. Food and drug administration (fda) to oversee the safety of food, drugs, medical devices, and cosmetics.a principal author of this law was royal s. Your device is exempted from 510(k) by regulation.
FREE 14+ Dental Medical Clearance Forms in PDF MS Word
+ 421 2 507 01 206. Štátny ústav pre kontrolu liečivsekcia klinického skúšania liekov a farmakovigilanciekvetná ul. Once the foreign manufacturer has received 510(k) clearance for the device, the foreign manufacturer may export his device to any u.s.
Once the foreign manufacturer has received 510(k) clearance for the device, the foreign manufacturer may export his device to any u.s. Food and drug administration (fda) to oversee the safety of food, drugs, medical devices, and cosmetics.a principal author of this law was royal s. The united states federal food, drug, and cosmetic act (abbreviated as ffdca, fdca, or fd&c) is a set of laws passed by the united states congress in 1938 giving authority to the u.s. Štátny ústav pre kontrolu liečivsekcia klinického skúšania liekov a farmakovigilanciekvetná ul. Tlačivo na hlásenie nežiaduceho účinku je na webovej stránke. Your device is exempted from 510(k) by regulation. + 421 2 507 01 206.