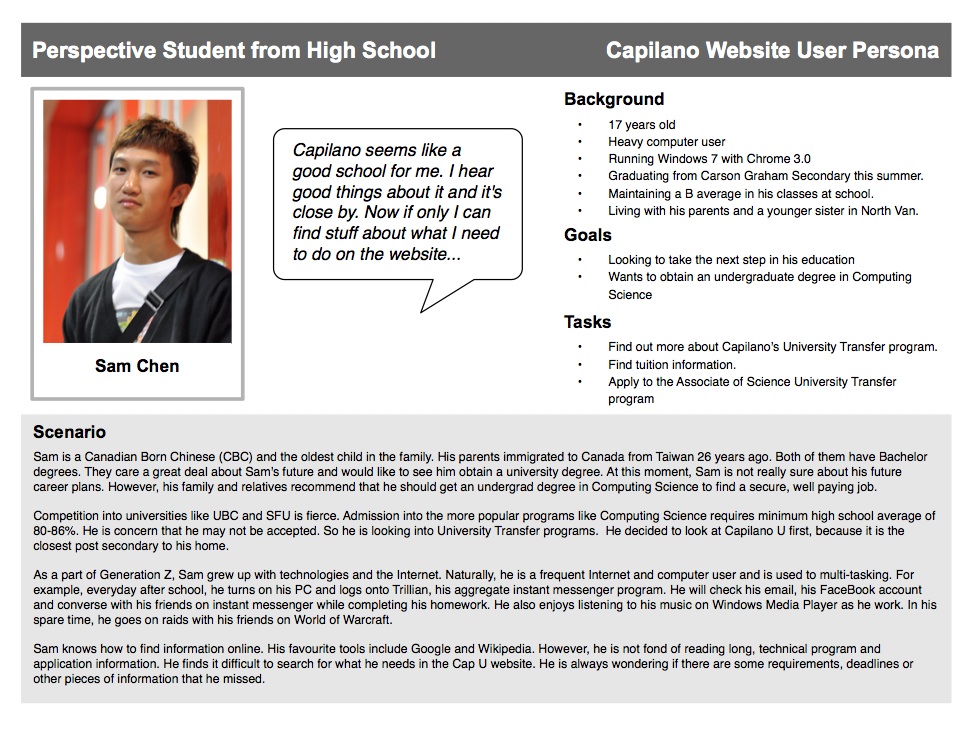
Product Dossier Template
Product dossier template - It should be preferably made in the english language or in an official language of an eu member state. The qp is expected to justify the controls in place on a scientific basis and record a risk assessment on a product specific basis6. We would like to show you a description here but the site won’t allow us. Browse google shopping to find the products you’re looking for, track & compare prices, and decide where to buy online or in store. Devices incorporate medicinal product under rule 14; The ectd dossier of the supporting documents or psurs , when applicable. Throughout the rmp template, ectd data/submissions should be read as ectd or ctd data/submission, corresponding to the type of submission to the competent authority. As a principle, such controls must provide confidence that the active substance is fit for purpose and will not negatively affect the safety and efficacy of the medicinal product. Dossier is an online platform for users to collect and curate scholarly materials, request and receive confidential letters of recommendations, and prepare for evaluations. Specific requirements for different types of initial
The mdr technical documentation template must be submitted to notified body or competent authority for review and approval. Faculty activity reporting drawing on millions of records through the interfolio data service, the interfolio faculty activity reporting module powers cvs, workload reports.
Simply Magazine Template Magazine Templates on Creative Market
Dossier is an online platform for users to collect and curate scholarly materials, request and receive confidential letters of recommendations, and prepare for evaluations. Browse google shopping to find the products you’re looking for, track & compare prices, and decide where to buy online or in store. Specific requirements for different types of initial
Customer Persona Example 3 Cooler Insights
As a principle, such controls must provide confidence that the active substance is fit for purpose and will not negatively affect the safety and efficacy of the medicinal product. Devices incorporate medicinal product under rule 14; Browse google shopping to find the products you’re looking for, track & compare prices, and decide where to buy online or in store.
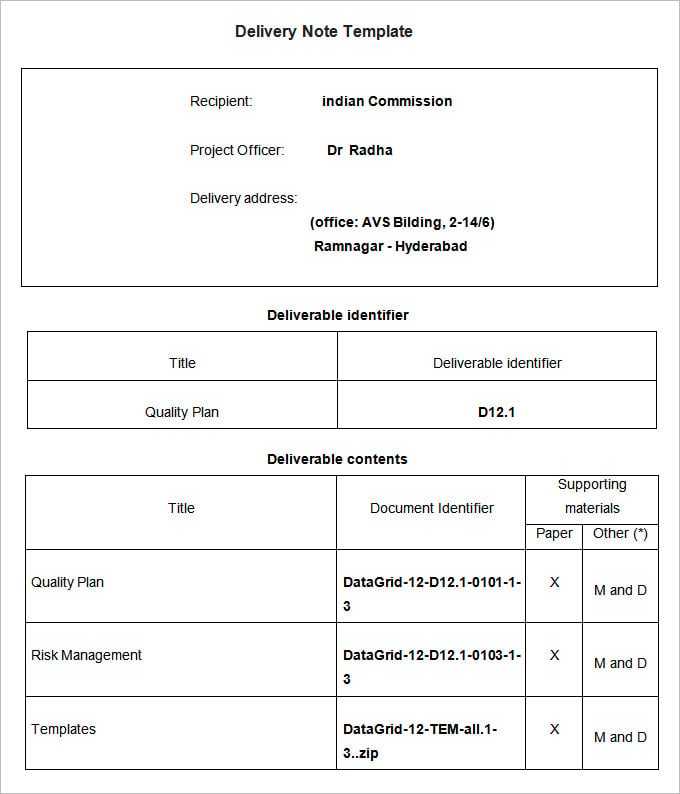
25+ Delivery Note Templates PDF, Docs, Word Free & Premium Templates
We would like to show you a description here but the site won’t allow us. Dossier is an online platform for users to collect and curate scholarly materials, request and receive confidential letters of recommendations, and prepare for evaluations. Browse google shopping to find the products you’re looking for, track & compare prices, and decide where to buy online or in store.
Company Profile Samples Find Word Templates
The qp is expected to justify the controls in place on a scientific basis and record a risk assessment on a product specific basis6. The mdr technical documentation template must be submitted to notified body or competent authority for review and approval. We would like to show you a description here but the site won’t allow us.
3 ways to get more value from your global value dossier
The ectd dossier of the supporting documents or psurs , when applicable. The qp is expected to justify the controls in place on a scientific basis and record a risk assessment on a product specific basis6. Devices incorporate medicinal product under rule 14;
Part 2 How to Write a Press Release for Your Fundraising Event
The qp is expected to justify the controls in place on a scientific basis and record a risk assessment on a product specific basis6. Faculty activity reporting drawing on millions of records through the interfolio data service, the interfolio faculty activity reporting module powers cvs, workload reports. The ectd dossier of the supporting documents or psurs , when applicable.
CJIS Security Policy Requirements Document — FBI
Browse google shopping to find the products you’re looking for, track & compare prices, and decide where to buy online or in store. It should be preferably made in the english language or in an official language of an eu member state. Specific requirements for different types of initial
Indesign Magazine Template Magazine Templates Creative Market
The mdr technical documentation template must be submitted to notified body or competent authority for review and approval. The qp is expected to justify the controls in place on a scientific basis and record a risk assessment on a product specific basis6. The ectd dossier of the supporting documents or psurs , when applicable.
The mdr technical documentation template must be submitted to notified body or competent authority for review and approval. Dossier is an online platform for users to collect and curate scholarly materials, request and receive confidential letters of recommendations, and prepare for evaluations. Devices incorporate medicinal product under rule 14; As a principle, such controls must provide confidence that the active substance is fit for purpose and will not negatively affect the safety and efficacy of the medicinal product. Faculty activity reporting drawing on millions of records through the interfolio data service, the interfolio faculty activity reporting module powers cvs, workload reports. Specific requirements for different types of initial We would like to show you a description here but the site won’t allow us. Throughout the rmp template, ectd data/submissions should be read as ectd or ctd data/submission, corresponding to the type of submission to the competent authority. It should be preferably made in the english language or in an official language of an eu member state. The ectd dossier of the supporting documents or psurs , when applicable.
The qp is expected to justify the controls in place on a scientific basis and record a risk assessment on a product specific basis6. Browse google shopping to find the products you’re looking for, track & compare prices, and decide where to buy online or in store.