Substance Template
Substance template - The attached qp declaration template provides a suitable means for documenting confirmation that the active substance manufacture complies with gmp requirements. Concerned active substance manufacturing sites the name of the active substance should be stated. A copy of the certificate of suitability is attached to this letter Active substance manufactured in accordance with the above active substance master file will no longer be supplied after [supply agreement termination date], replacement of the active substance master file by certificate of suitability, [cep no]. The format of the qp declaration template is in five parts (parts a to e).
Examples of different Hazardous substances, New Zealand (NZ). Stock
The format of the qp declaration template is in five parts (parts a to e). A copy of the certificate of suitability is attached to this letter Active substance manufactured in accordance with the above active substance master file will no longer be supplied after [supply agreement termination date], replacement of the active substance master file by certificate of suitability, [cep no].
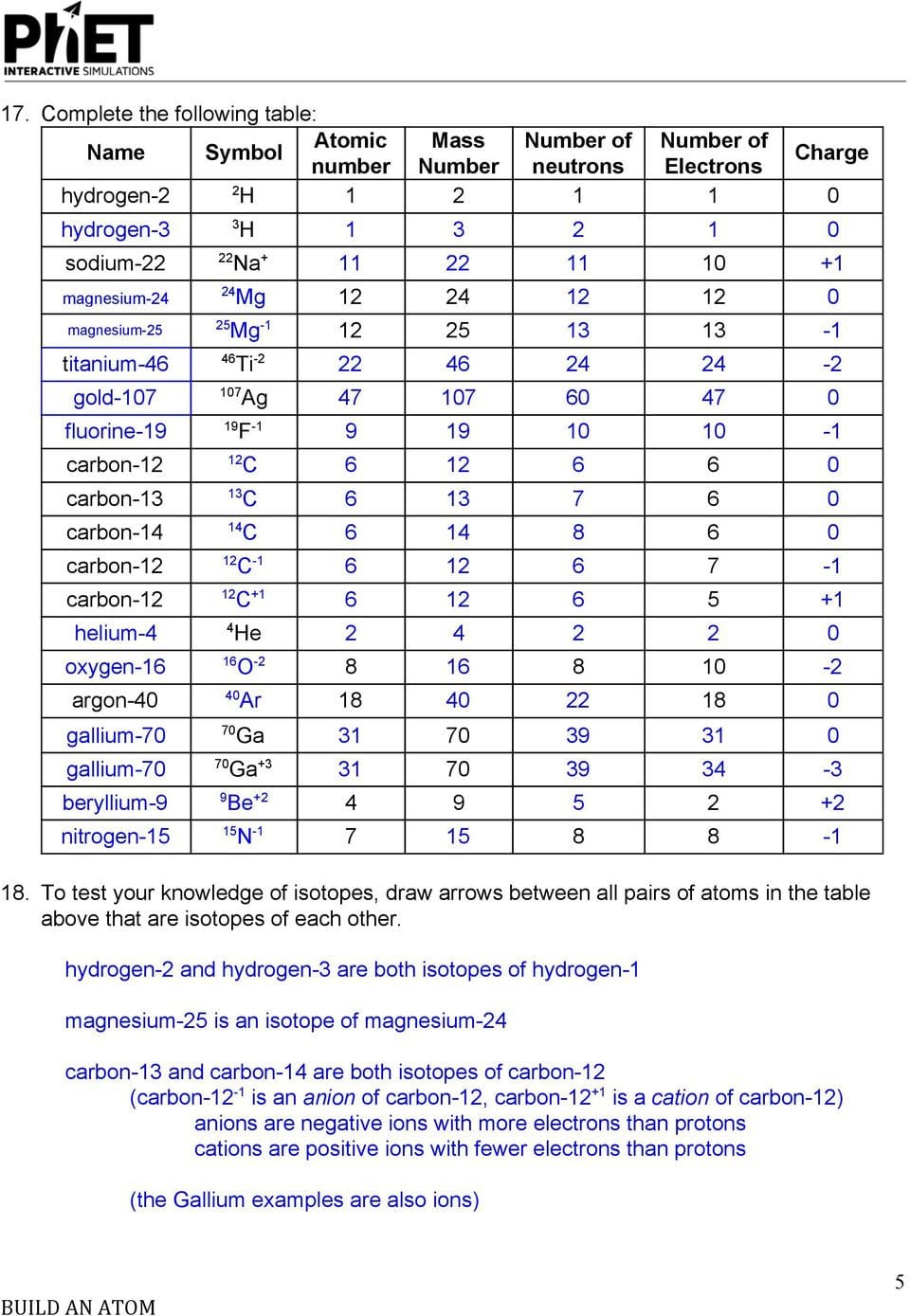
Answer Key Build An Atom Part I Atom Screen Build An Atom —
Concerned active substance manufacturing sites the name of the active substance should be stated. Active substance manufactured in accordance with the above active substance master file will no longer be supplied after [supply agreement termination date], replacement of the active substance master file by certificate of suitability, [cep no]. The format of the qp declaration template is in five parts (parts a to e).
Tool Kit Research Quality and Compliance The University of Utah
Concerned active substance manufacturing sites the name of the active substance should be stated. Active substance manufactured in accordance with the above active substance master file will no longer be supplied after [supply agreement termination date], replacement of the active substance master file by certificate of suitability, [cep no]. A copy of the certificate of suitability is attached to this letter
Australian Handwriting Worksheets Victorian Modern Cursive —
Concerned active substance manufacturing sites the name of the active substance should be stated. The attached qp declaration template provides a suitable means for documenting confirmation that the active substance manufacture complies with gmp requirements. Active substance manufactured in accordance with the above active substance master file will no longer be supplied after [supply agreement termination date], replacement of the active substance master file by certificate of suitability, [cep no].
Clinical Counselor Resume Samples QwikResume
Concerned active substance manufacturing sites the name of the active substance should be stated. Active substance manufactured in accordance with the above active substance master file will no longer be supplied after [supply agreement termination date], replacement of the active substance master file by certificate of suitability, [cep no]. The format of the qp declaration template is in five parts (parts a to e).
Substance Abuse Counselor Intern Resume Samples QwikResume
A copy of the certificate of suitability is attached to this letter The format of the qp declaration template is in five parts (parts a to e). The attached qp declaration template provides a suitable means for documenting confirmation that the active substance manufacture complies with gmp requirements.
Treatment Planning Mastering Competencies 2nd edition YouTube
The attached qp declaration template provides a suitable means for documenting confirmation that the active substance manufacture complies with gmp requirements. The format of the qp declaration template is in five parts (parts a to e). Active substance manufactured in accordance with the above active substance master file will no longer be supplied after [supply agreement termination date], replacement of the active substance master file by certificate of suitability, [cep no].
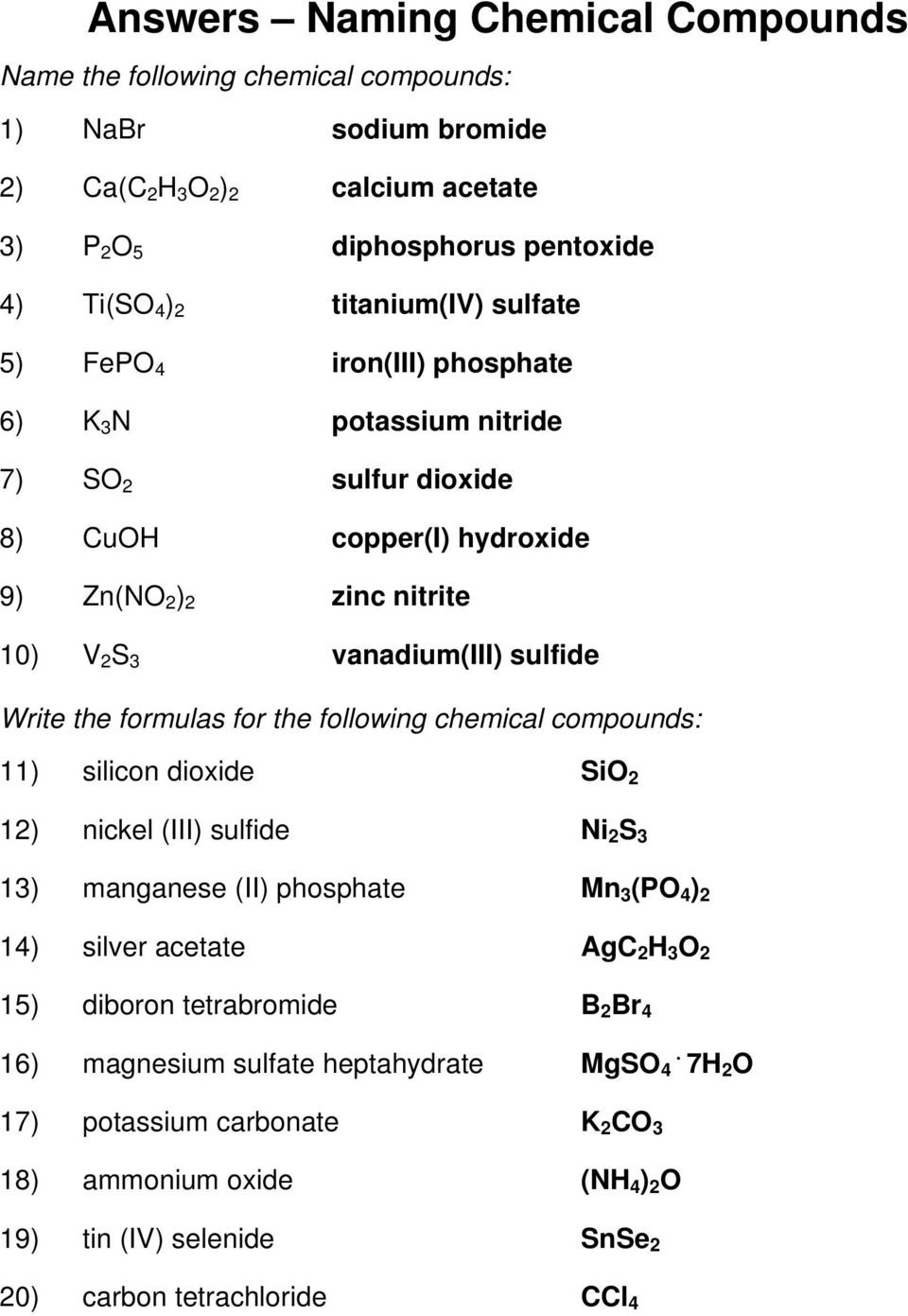
Naming Ionic Compounds Answer Key Pdf —
The attached qp declaration template provides a suitable means for documenting confirmation that the active substance manufacture complies with gmp requirements. Active substance manufactured in accordance with the above active substance master file will no longer be supplied after [supply agreement termination date], replacement of the active substance master file by certificate of suitability, [cep no]. A copy of the certificate of suitability is attached to this letter
The format of the qp declaration template is in five parts (parts a to e). Active substance manufactured in accordance with the above active substance master file will no longer be supplied after [supply agreement termination date], replacement of the active substance master file by certificate of suitability, [cep no]. A copy of the certificate of suitability is attached to this letter The attached qp declaration template provides a suitable means for documenting confirmation that the active substance manufacture complies with gmp requirements. Concerned active substance manufacturing sites the name of the active substance should be stated.